Concentration profile in electrodialysis:
Because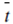 > t, i.e., the transport number of counter ions by membrane is more than in solution, the ion flux in solution will be lower compared to their flux by the membranes. The electrolytic movement of counter ions up to the membrane surface will be not sufficient to meet the faster rate of its migration through the membranes. This difference will lead to depletion of counter ions on the dialysate side adjacent to membrane and a build up of counter ions on the brine side adjacent to the membranes. This is known as concentration polarization and is schematically depicted in Figure.
> t, i.e., the transport number of counter ions by membrane is more than in solution, the ion flux in solution will be lower compared to their flux by the membranes. The electrolytic movement of counter ions up to the membrane surface will be not sufficient to meet the faster rate of its migration through the membranes. This difference will lead to depletion of counter ions on the dialysate side adjacent to membrane and a build up of counter ions on the brine side adjacent to the membranes. This is known as concentration polarization and is schematically depicted in Figure.
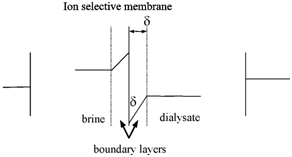
Figure: Concentration profile in electrodialysis
With rise in current density, the concentration polarization becomes steeper and at sufficiently high current densities, a counter ion migration at layer adjacent to the membrane within the dialysate approaches zero. Because there will not be any ions to carry the current, any addition current through the membranes will be carried through the hydrogen and hydroxyl ions formed through the ionization of water at the interface. The current at that this water splitting starts to occur is known the limiting current density (ilim), since any further increase in current density would cause loss of coulomb efficiency , increased electrical resistance and changes in the pH of the solutions.
The limiting current density is described by setting Co = 0 in Eq. in which case
ilim = C D Z/δ (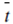 - t )
- t )
While C,D,Z and δ indicates concentration of the ion in the bulk dialysate solution, diffusivity in solution, electronic charge and thickness of the boundary layers, respectively.