Standard potentials
A redox reaction takes place in two halves in an electrochemical cell. Electrons are liberated through the oxidation half reaction at one electrode and pass via an electrical circuit to other electrode in which they are employed for the reduction. The cell potential E is the potential variation among the two electrodes needed to balance the thermodynamic tendency for reaction, as a result the cell is in equilibrium and no electrical current flows. E is associated to the molar Gibbs free energy change in the entire reaction according to
?G = - n F E
In which F is the Faraday constant that is (9.6485×104 C mol-1) and n the number of moles of electrons passed per mole of reaction.
Table 1. Some standard electrode potentials in aqueous solution at pH=0 and 25°C
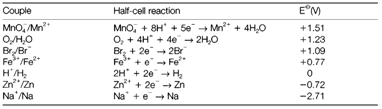
It is helpful to think of the cell potential as the variation between the potentials related with the two half-cell reactions, even though these are not separately measurable. The Standard electrode potentials are the half-cell potentials calculated against a hydrogen electrode, in which the half-cell reaction is as follow:
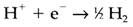
All reagents being under basic conditions (unit pressure and activity and). Some values are displayed in Table 1 for species in aqueous solution. Through convention, tabulated potentials consider to reduction reactions, with electrons on the left side as in the above equation. Only variations in electrode potential are important, the absolute values having no meaning apart from in comparison with the H+/H2 potential (zero by definition).