Nonstandard conditions
The electrode potential of a couple under nonstandard conditions can be calculated from the Nernst equation:
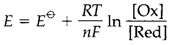
where [Ox] and [Red] are the activities of the species included; it is a general approximation, particularly in dilute solutions, to suppose that these are similar as the molar concentrations. With n=1 at 298 K, a factor of 10 variations in the activity changes E through 0.059 V.
While a reaction involves H+ or OH- ions, these must be involved in the Nernst equation to guess the pH dependence of the couple. So for the half-cell MNO-4/MN2+ reaction displayed in Table 1. a issue of [H+]8 should be involved in the [Ox] term, leading to a reduction in potential of (8/5)×0.059=0.094 V per unit raise in pH. pH changes might also have a more subtle affect by altering the species involved. For instance, in alkaline solution the ion Mn2+ precipitates as Mn(OH)2. Standard potentials at pH=14 consider to reactions written with OH- rather than H+.
Potentials may also be strongly affected by complex formation. Generally, any ligand that complexes more strongly through the higher oxidation state will decrease the potential. For instance, cyanide (CN-) complexes much more strongly with Mn3+ than with Mn2+, and at unit activity decreases the Mn3+/Mn2+ potential from its standard value of +1.5 V to +0.22 V. Alternatively, the potential get increases if the lower oxidation state is more strongly complexed.