Direction of Reaction
By Comparing two couples Ox-Red, a more positive potential meaning of that is the subsequent species Ox is a stronger oxidizing agent. So from Table 1 Br2 is a stronger oxidizing agent than Fe3+ and will so oxidize Fe2+, the results being Br- and Fe3+. On the other hand, a lower (more negative) potential means that the subsequent Red is a stronger reducing agent. So zinc metal is a stronger reducing agent than dihydrogen, and will decrease H+:
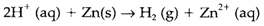
The equilibrium constant K for such type of a reaction can be calculated from
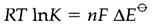
In which R is the gas constant, T the temperature in kelvin, .....
the variation in the two electrode potentials and n the number of electrons in every half reaction: this must be similar for both half reactions in a balanced equation. In Equation 2 n=2, that gives K around 1024 at 298 K for this reaction. Like the potential of a single half-cell is not measurable, that's why an equilibrium constant based on a single potential has no meaning.