Diagrammatic representations
A Latimer picture depicts the standard electrode potentials related with the dissimilar oxidation states of an element, as demonstrated in Fig. 1 for manganese. Potentials not given explicitly can be mesured by using Equation 1 and taking careful account of the number of electrons involved. So, the free energy alter for the Mn3+/Mn reduction is the sum of those for Mn3+/Mn2+ and Mn2+/Mn. From Equation 1 so:
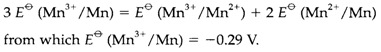
Fig. 1. Latimer diagram for Mn at pH=0.
In a Frost or oxidation state figure each oxidation state (n) is allocated a volt equivalent equal to n times its E? value as respect to the element. The potential E? in volts among any two oxidation states is equivalent to the slope of the line among the points in this diagram. Steep positive slopes depict strong oxidizing agents, the steep negative slopes strong reducing agents. Frost figures are suitable for depicting the comparative redox properties of elements.
Frost diagrams also give a visual guide to when disproportionation of a species is supposed. For instance, in Fig. 2 the Mn3+ state at pH=0 is found above the line created through joining Mn2+ with MnO2. It follows that than MnO2/Mn3+ the Mn3+/Mn2+ potential is more positive, and disproportionation is supposed:
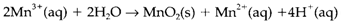
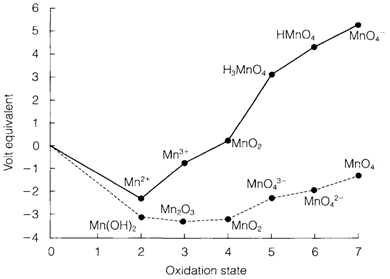
Fig. 2. Frost diagram for Mn at pH=0 (solid line) and pH=14 (dashed line).
This reaction's equilibrium constant can be mesured by noting that it is made up from the half reactions for Mn3+/Mn2+ and MnO2/Mn3+ each with n=1, and has E?= 1.5- 0.95=0.55V from Fig. 1. giving K=2×109. The states MnV and MnVI are likewise unstable to disproportionation at pH=0, where at pH=14, also depict in Fig. 2. only MnV will disproportionate. Latimer and Frost diagrams show similar information but in a dissimilar way. While interpreting electrode potential data, either in graphical or numerical form, it is significant to keep in mind that a single potential in isolation has no meaning.