Liquid-Junction Potentials:
When two different electrolyte solutions are brought into contact an electrical potential difference arises at the zone of contact. This potential make difference is termed liquid- junction potential (Ej) or diffusion potential. It is caused because of the diffusion of ions from regions of higher to lower concentrations depending on the concentration gradient and the individual mobility of each ion. Various types of liquid-junction potentials are possible; one simple type of Ej is illustrated below.
Suppose we could prepare a quiet interface between two solutions containing the same electrolyte but at different concentrations, such as 0.1 M HCl and 0.01 M HCl. On making a contact of these two solutions, immediately, both H+ and Cl- ions diffuse from left to right due to concentration gradient. However, hydrogen ions move much more rapidly than do chloride ions and are indicated by the longer arrow for H+ in figure. Thus, H+ outruns Cl- and there is a slight tendency for a charge separation with the right side of the junction acquiring a positive charge and the left side a negative charge.
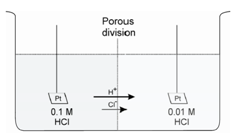
Figure: Liquid-Junction Potential
The liquid-junction potentials may vary over a considerable range depending on the conditions of the cell. These potentials could be minimized through using a salt bridge containing a concentrated electrolyte solution of cation and anion having comparable mobilities. For example, potassium and chloride ions have comparable mobilities, and salt bridges of saturated aqueous potassium chloride, with agar gel, are widely used to minimize liquid junction potentials.