Development of Electrode Potential:
Consider a metal M that is placed in a solution containing its ions Mn+. The metal may be looked upon as being composed of metal ions and electrons. The phases, the metal and the solution contain metal ions Mn+ but the activity of Mn+ in the metal will be different from that in the solution and distribution of metal ions takes place in the two phases in order to get the position of equilibrium. We will consider, for two types of metals, one less active, for example copper, and the other more active say zinc by placing them in contact with their salt solutions.
i) In case one, when a less active metal, say a piece of copper is placed in a solution of copper sulphate. A few of the copper ions might deposit on the copper metal and accepting electrons from the metal conduction band and leaving the metal with a small positive charge and the solution with a small negative charge.
ii) In the second situation with a more active metal it will be the other way around, a few metal ions from the metal surface may pass into the solution phase, giving the metal a small negative charge and the solution a small positive charge.
Within both cases, the positive and negative charges will be located at the surface of the metal solution phases, called the interface.
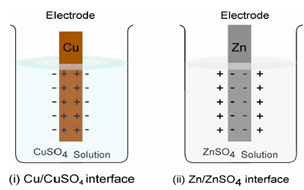
Figure: Metal - Metal ion Interface
As an output an electrical double layer is build along with a corresponding potential difference among metal and solution, and is called the electrode potential.