Metal Surface Showing Arrangement of Micro-cells:
The surface of any metal is a composite of an extremely large number of micro-electrodes, as described in Figure. In sequence for corrosion to occur, a micro-cell must also be linked by a few conducting path external to the metal. Commonly the external connection is given through water or an aqueous solution and the cells generate a current, permitting the chemical reactions responsible for corrosion to proceed.
Let consider iron in water again. iron would go into solution as Fe++ ions until the difference in potential between the positively-charged solution and the negatively-charged metal stopped the iron ions from leaving the surface if the surface of the iron and the water solution were uniform,. In practice, by, impurities and imperfections (for instance, oxide coatings) lead to preferential removal of metal from certain categories of the surface or potential differences arise as in the two metal system. The corrosion cells, changing as surface and solution differences change, cause common overall corrosion. Pitting results if the cells do not shift.
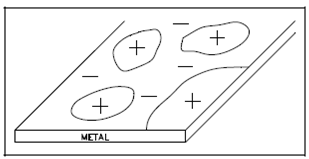
Figure: Metal Surface Showing Arrangement of Micro-cells