Galvanic Cells:
In a galvanic cell, the system comprises of two electrodes (half cells), processes occur whereby the energy associated with chemical reactions is converted into electrical energy. In this way we can obtain a useful system upon which meaningful measurements can be performed. A single such cell called as Daniell cell is illustrated in Figure.
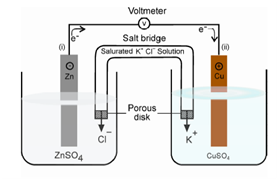
Figure: A Galvanic cell (Daniell cell)
The solutions of the two half-cells are connected with a salt bridge, say an inverted U-tube containing a solution of potassium chloride with agar gel. The zinc strip immersed in zinc sulphate constitutes zinc electrode, one half cell, and the copper strip immersed in copper sulphate constitutes copper electrode, the second half cell. The combination of these two half cells will give the complete cell. Such a cell was invented by Luigi Galvani. It was several of such cells built to supply electrical energy before electrical generators were available.
When the circuit is closed, the cell operates spontaneously. The copper has a higher positive potential than the zinc, or electrons flow from zinc to copper in the outer circuit through the wire. A flow of electrons is known an electric current. Conventionally, the flow of current is considered in a direction opposite to that of electrons, based on the consideration of potential, such that from a region of more positive potential to a less positive (or more negative) potential.