Miniature cells and batteries:
In recent years, cells and batteries-especially cells-have become available in many various sizes and shapes besides the cylindrical cells, transistor batteries and lantern batteries. These are used in cameras, watches, and several other microminiature electronic products.
Silver-oxide types
Silver-oxide cells are usually made into button shapes, and can fit inside even a small wristwatch. They come in several sizes and thicknesses, all with the similar appearances. They provide 1.5 V, and offer excellent energy storage for the weight. They also form a flat discharge curve, like the one shown in the graph of Figure given below. The previously described zinc-carbon and alkaline cells and batteries have a current output that declines with time in a steady fashion, as shown in Figure given. This is known as a declining the discharge curve.
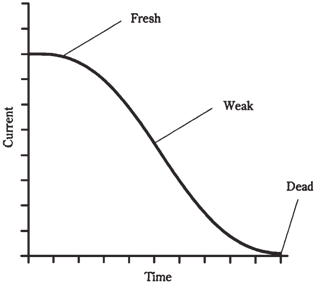
Figure-- A declining discharge curve.
Silver-oxide cells are stacked to construct the batteries. A number of these miniature cells, one on top of the other, may give 6 V or 9 V for a transistor radio or other light-duty electronic device. The resulting battery is of the size of an AAA cylindrical cell.
Mercury types
Mercury cells, called as mercuric oxide cells, have advantages identical to silver-oxide cells. They can be manufactured in the same general form. The main difference, not of significance often, is a lower voltage per cell: 1.35 V. If 6 of these cells are stacked to make a battery, the resulting voltage will be around 8.1 V rather than 9 V. One additional cell can be added to stack, yielding around 9.45 V.
There has been a little decrease in the popularity of mercury cells and batteries in the recent years. This is due to the fact that mercury is very toxic. When mercury cells and batteries are dead, they should be discarded. Gradually the mercury, a chemical element, leaks into the soil and ground water. Mercury pollution has become an important concern in places which might really surprise you.
Lithium types
Lithium cells have become popular as the early eighties. There are variations in the chemical makeup of these cells; they all contain lithium, a light, reactive metal. Lithium cells are made to supply 1.5 V to 3.5 V, depending upon the special chemistry used. This cell, like their silver oxide cousins, is stacked to make batteries.
The 1st applications of lithium batteries were in memory backup for electronic microcomputers. Lithium cells and batteries posses superior shelf life, and they can last for years in quite low current applications such as memory backup or the powering of a dig- ital liquid-crystal-display watch or clock. These cells provide energy capacity per unit volume that is greater than other types of electrochemical cells.
The cells and batteries of lithium are used in low power devices that which operate for a long time without the replacement of power source. Heart pacemakers and security systems are 2 examples of this type of applications.