Ions
If the atom posses more or less electrons than the neutrons, that atom acquires an electrical charge. A shortage of electrons results in positive charge; an excess of electrons gives a negative charge. The element's identity remains the same, no matter how large the excess or shortage of electrons. In extreme case, all the electrons can be removed from an atom, leaving the nucleus. But it would still represent the same element as it would if it had all its electrons.
A charged atom is termed an ion. When a substance has many ions, the material called as ionized.
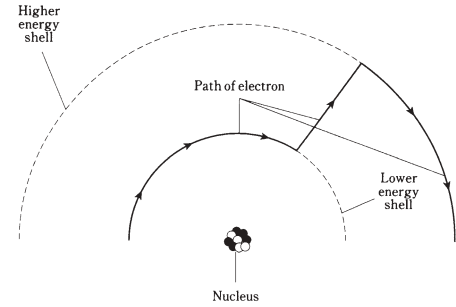
Figure: Electrons move around the nucleus of an atom at certain levels corresponding to different energy states. This is simplified drawing, showing an electron gaining energy.
An example of an ionized substance is atmosphere of the earth at high altitudes. The ultraviolet radiation from sun, and high-speed subatomic particles from space, result in gases' atoms being stripped of electrons. The ionized gases tend to be found in layers at some altitudes. These layers are responsible for long distance radio communications at certain frequencies.
Ionized materials conduct electricity quite well, even if the substance is not a good conductor normally. Ionized air makes it possible for a lightning stroke to occur, for instance. The ionization, due to a powerful electric field, occurs along a jagged, narrow channel, as you have seen. After lightning flash, nuclei of the atoms attract quickly stray electrons back, and the air becomes neutral electrically again.
An element can be an ion and an isotope both different from the usual isotope. For example, an atom of carbon mau have 8 neutrons rather than the usual 6, hence being the isotope C14, and it may have been stripped of an electron, giving it a positive unit electric charge and making it an ion.