Polarity and solvation
A solvent is a liquid medium within which dissolved substances are termed as solutes. Solvents are helpful for storing substances that would or else be in inconvenient states (example gases) and for facilitating reactions that would otherwise be tough to carry out (example ones involving solids). The chemical and physical features of a solvent are significant in controlling what substances dissolve simply, and what types of reactions can be performed. The chemical with the physical state of solutes may be changed by interaction with the solvent. A list of helpful solvents is given in Table 1.
The most significant physical property of a solvent is its polarity. Molecules with large dipole moments like ammonia and water form polar solvents. The macroscopic demonstration is the dielectric constant (εr), the issue by which electrostatic forces are weakened in comparison with those in a vacuum. For instance, in water εr=82 at 25°C, and so attractive forces among cations and anions will be weaker by this factor.
At a microscopic level, solutes in polar solvents go through strong solvation. For instance, the Born model supposes that the Gibbs free energy of an ion with charge q (in Coulombs) and radius r will be altered in the solvent compared with the gas phase by an amount
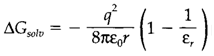
Table 1. Properties of some solvents, depicting normal melting and boiling points (MP and BP, correspondingly), dielectric constant (εr, at 25°C or at the boiling point if that is lower), and donor and acceptor numbers (DN and AN, respectively)
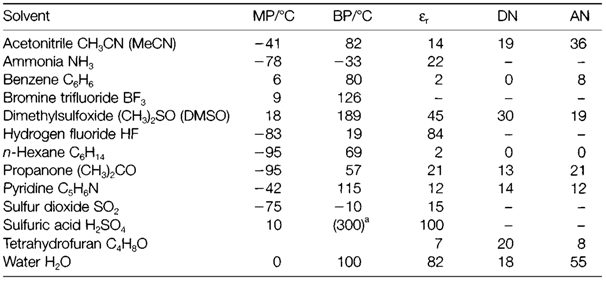
This approximation of the solvation energy is highly approximate, as it supposes that the solvent can be treated like a continuous dielectric medium on a microscopic scale. However, it gives a rough guide that is helpful in interpreting solubility trends.
Actually, solvation includes donor-acceptor interactions, which might not be simply electrostatic in nature, so that neutral molecules might also be strongly solvated. Solvent molecules are ordered round the solute, not only within the primary solvation sphere but (particularly with ions) influencing more distant molecules. So, Solvation generates a decrease in entropy, that can be substantial with small highly charged ions and contributes to solubility, acid-base strength and complex formation trends.
Nonpolar solvents like hexane have molecules with little or no dipole moment and low dielectric constants. They are usually better at dissolving nonpolar molecules and for carrying out reactions where no ions are involved. The molecules interact mainly by van der Waals' forces (see Topic C10). Nonpolar media are usually poor solvents for polar molecules since the weak intermolecular forces cannot compete with the stronger ones in the pure solute. Likewise, nonpolar solutes cannot compete with the strong intermolecular forces within a polar solvent and so may not be extremely soluble. These generalizations have several limitations. Ionic substances can dissolve in solvents of lower polarity if the ions are well solvated by suitable donor and acceptor interactions. Like the electrostatic forces among solvated ions remain comparatively strong, though, they tend to form ion pairs. Even though liquid ammonia (εr=22) is a good solvent for some ionic compounds, ion pairing is very much commoner than in water (εr=82).