Ion-transfer solvents
Ammonia, Water and other protic solvents go through a reaction termed as autoprotolysis:
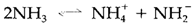
Even though the equilibrium constants may be small (approximately 10-30 for ammonia) the probability of such type of reactions leads to a definition of acids and bases based on a solvent system. An acid is the positive species created ( NH4+ in the above instance) or any solute that gives increment to it; likewise, a base is the negative species (NH2-) or anything producing it in solution. By protic solvents this corresponds to the acids and bases's Brønsted definition. The instances in Table 2 depict that something acting as an acid in one solvent can be a base in other.
Aprotic solvents do not comprise the transferable H+ but some other ion like halide or oxide can be involved. Table 2 depicts the instance of BrF3, which goesthrough some autoionization with F- transfer. Substances dissolving to give F- ions that are act as bases, and Lewis acids which can react with F- act as acids:
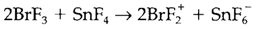
The solvent system corresponds to the Lux-Flood acid/base, in oxide melts definition: an oxide ion acceptor an acid and an oxide donor is a base. In the reaction
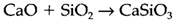
the silica acidic is basic, and calcium oxide the. Lux-Flood acidities of oxides are significant in reactions occurring in silicate melts, for instance in glass manufacture. The values correlate well with other aspects of acid-base behaviour for instance that manifested in aqueous chemistry. Acidity of EOn/2 usually rises with the oxidation state n and is larger for smaller ions En+ and for non-metallic elements. Strongly basic oxides which work as oxide donors involve Na2O and CaO; acidic oxides acting as oxide acceptors involve B2O3 and P2O5.