Influence of defects
All solids contain defects in which the regularity of the ideal periodic lattice is broken. Plane and Line defects (dislocations, grain boundaries, etc.) are significant for mechanical properties but it is point defects that are most important for electrical properties. They involve
- interstitials or atoms in positions not generally occupied;
- impurities either accidentally introduced or present as deliberate doping.
- atoms or vacancies missing from regular lattice positions;
Defects which introduce extra electrons or that give missing electrons or 'holes', have a large effect on electronic conduction in nonmetallic solids. Several semiconductor devices use doped or extrinsic semiconductors rather than the intrinsic semi conduction of the pure material. Doping Si with P replaces some of the tetrahedrally bonded Si atoms in the diamond lattice with P. Each replacement gives one extra valence electron, which needs only a small energy to escape into the CB of silicon. This is an n-type emiconductor. Alternatively, replacing an Si atom with Al gives a missing electron or 'hole', that may move in the VB giving a p-type semiconductor. Some other sorts of nonmetallic solid can be doped, particularly compounds of transition metals, that have variable oxidation states. So slight reduction of TiO2 introduces electrons and provides n-type behavior. Likewise, oxidation of NiO removes several electrons and it becomes a p-type semiconductor.
Despite of providing electrons, atoms in defect sites might themselves be mobile and so give ionic conduction in a solid. Ionic compounds like NaCl have high conductivity in their molten form and such type of conductivity is significant for the manufacture of aluminum through electrolysis of molten cryolite (Na3AlF6). Though, In the most solids ionic conduction is much lower and increases largely from defects. Interstitial ions and vacancies in ionic compounds must take place in combinations that give overall electrical neutrality. Two significant combinations are Schottky defects in which there is an equal concentration of cation and anion vacancies and Frenkel defects where vacancies of one ion are balanced by interstitials of similar kind. For instance NaCl has predominantly Schottky defects and silver halides (AgCl and AgBr) mainly Ag+ Frenkel defects. Both interstitial vacancies and ions may be mobile and so contribute to ionic conduction. Doping with ions of dissimilar charge may change the defect concentrations and so the conductivity.
For instance if AgBr is doped with a small concentration of CdBr2, each Cd2+ replaces two Ag+ ions. So, the concentration of Ag+ vacancies is increased and that of interstitials decreased. Like the interstitials are more mobile than the vacancies in AgBr, the primary influence of doping is to decrease the ionic conductivity. Though, as the concentration of Cd2+ is increased the vacancies become suitably numerous to dominate the conduction process, and so conductivity increases again.
Some solids, termed as fast ion conductors depict a degree of ionic conduction that is comparable to that of the molten form and that cannot be attributed to low concentrations of defects. For instance above a transition temperature of 146°C, AgI accepts a structure with a body-centered cubic array of I-. The Ag+ ions move independently among a variety of sites in which they have almost equal energy. One cannot think severely of defects in a case like this, rather it is the nonexistence of a unique ordered structure that gives rise to high ionic conductivity. Anions are mobile at temperatures well under the melting point in some compounds with the fluorite structure, like PbF2 and ZrO2. The oxide ion conductivity of ZrO2 can be increased by doping with CaO or Y2O3. So, in Ca0.1Zr0.9O1.9 (consistent with the ionic charges Ca2+, Zr4+ and O2-) the ratio of anions to cations is less than the value 2:1 needed for the normal ZrO2 lattice, so that oxygen vacancies are exist. Doped ZrO2 is employed as a 'solid electrolyte' in electrochemical sensors and in fuel cells. One significant application is in sensors which measure the O2 concentration of exhaust gases from automobile engines and is employed in conjunction with 'catalytic converters' for removing pollutants.
Two platinum electrodes are placed on reverse faces of a sample. Oxygen gas reacts at one electrode as per the
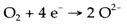
Oxide ions pass via the solid and the opposite reaction takes palce at the other electrode. A potential variation is developed among the two electrodes that depend on the ratio of O2 partial pressures on each side.