Donor and acceptor properties
Most polar solvents have Lewis base or donor properties resultant from lone-pair electrons. Good donor solvents involve water, ammonia and pyridine and are efficient at solvating cations and other Lewis acids.
Acceptor or Lewis acid behavior is significant for solvating anions, and products from empty orbitals or from hydrogen bonding. Donor and acceptor numbers have been described through measuring the force of interaction among solvent molecules and the 'standard' donor (OPCl3) and acceptor (SbCl5) molecules, correspondingly. Values are displayed in Table 1. and can provide a helpful guide even though they ignore many particular details of the interaction, and particularly make no distinction among 'soft' and 'hard' character. As an instance of this restriction, benzene is listed as having no
Table . Some ion-transfer solvents, with the characteristic solvent-system acid and base species, and other instances of acids and bases
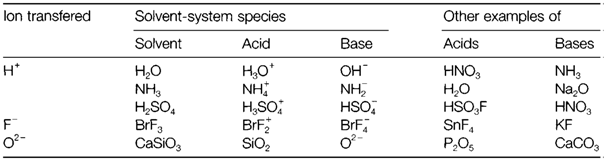
considerable donor strength, yet will dissolve silver perchlorate AgClO4 due to a strong 'soft' donor-acceptor interaction among Ag+ and a benzene molecule. In several cases a donor-acceptor interaction might be only the first step within a more substantial solvolysis reaction. These reactions are general with nonmetal oxides and halides in water and ammonia; for instance,
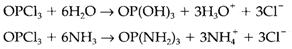
An instance of the variety of results formed in distinct donor solvents is provided by the reactions of FeCl3, in which S denotes a coordinated solvent molecule:
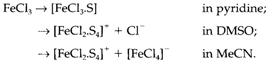
These variations are thought to product from the lower polarity of pyridine compared with the other two solvents and the better solvation of small ions like Cl- in DMSO compared with MeCN.