Effect of Complexing Agents:
Within case of complexation of metal ion along with the complexing agent the electrode reaction proceeds in two steps
MXp (n-pb)+ ↔Mn++ pX-b ... (8.14)
M n++ ne-+ Hg ↔ M (Hg) ... (8.15)
and E1/2 of the metal ion is shifted to more negative value compared to the reduction of simple metal ion in the non-complexing medium. The expression for the electrode potential for such system is given as
(E 1/2 )= Eo + (0.05915/n) log Kinstab- (0.05915/n) log [Xb -] p ... (8.16)
where p is the co-ordination number of the complex formed and Xb- is the complexing agent. p is determined by taking E1/2 values with varying concentrations of complexing agent.
(E 1/2 )b-(E 1/2 )simple =0.05915/n log Kinstab -p 0.05915/n log[Xb - ] ... (8.17)
If a graph is drawn between log[X] and E1/2 a line with the slope equivalent to [- p 0.05915/n] will be obtained from which
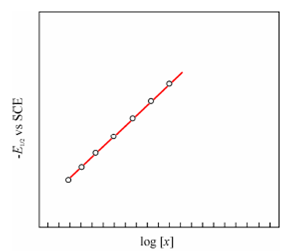
Figure: Log plot
'p' is determined. Once p is known the instability constant of the complex ion is determined while using the Eq. (8.17). A shift of E1/2 of metal ion through complexation is meaningful for quantitative analysis of samples holding more than one metal ion to eliminate interfering effect of one metal ion upon another or promote separation of E1/2 of metal ions in a mixture for simultaneous determinations.