E2 elimination process:
The E2 elimination is stereo specific, along with elimination taking place in antiperiplanar geometry. The figure depict that the four atoms included in the reaction are in a plane with the H and Br on opposite sides of the molecule.

Figure: Relative geometry of the atoms involved in the E2 elimination mechanism.
The cause for this stereospeci?city can be described by using the orbital diagrams. In the transition state of this reaction, the C-H and C-Br σ bonds are in the procedure of breaking. As they do so, the sp3 hybridized orbitals that were employed for these σ bonds are changing into p orbitals that start to interact with each other to make the eventual π bond. For all this to occur in the one transition state, an antiperiplanar arrangement is necessary.
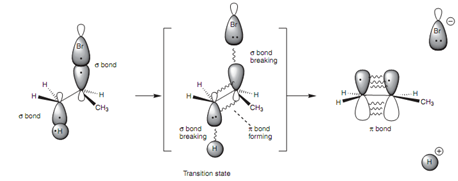
Figure: Orbital diagram of the E2 elimination process.