Disinfection by Chlorine:
Chlorine and its compounds are most widely used as disinfectant due to their wide availability at economical rate. It also easily kills pathogens and leaves residual effect for future recontamination. However, if applied in excess it may not only make water unpalatable but also cause toxicity in water.
It is found in three forms, i.e.
(a) Liquefied chlorine,
(b) Chlorine solution (NaOCl), and
(c) Powdered chlorine in the form of chlorinated lime (commonly known as bleaching powder).
The form in which chlorine should be applied in water treatment is governed by several factors such as the quantity of water to be treated, the cost and availability of chemicals, the equipment needed for its application and the skill required for operation and control.
It is a powerful oxidising agent, which when dissolved in water yields the following reaction:
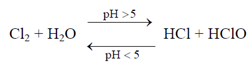
which is followed by the secondary reaction.
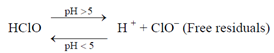
Hypochlorous acid, HClO, is the more effective disinfectant, the chlorite ion, ClO-, being far less effective. The dissociation of HClO is suppressed at acidic pH range, the residual being all HClO at pH5 and below, about half HClO at pH 7.5 and all ClO- at pH 9 (Figure 6).
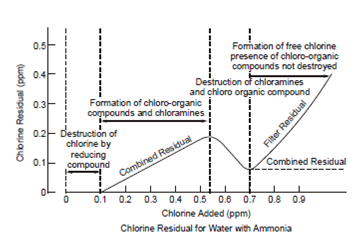
Figure: Chlorine Reactions in Water
If ammonia is present in the water, the reaction is:
NH3 + HClO → NH2Cl + H2O (at pH 4.5 - 8.5 and alone at pH above 8.5) Monochloramine
NH2Cl + HClO → NH1Cl2 + H2O/ dichloramine (at pH 4.5 - 8.5)
NH2Cl + HClO → NH3 + H2O/trichloramine (alone at pH less than 4.4)
The chloroamines are the combined residuals. They are more stable than the free residuals but less effective as disinfectants. For a given kill rate with constant residual, the combined form required over hundred times the contact time required by the free residual. Alternatively for a given contact time, the combined residual concentration must be twenty five times the free residual concentration to give the desired killing.