Silver-Silver Chloride Electrodes:
This electrode is similar to the one given above. That consists of a silver wire or a silver plated platinum wire coated along with a thin layer of silver chloride, immersed in a solution of potassium chloride of a known concentration. It could be represented through:
AgCl (sat.), KCl (x M) | Ag ... (2.29)
and the half-cell reaction is :-
AgCl (s) + e → Ag (s) + Cl¯ ... (2.30)
Normally this electrode is prepared with saturated potassium chloride solution and its potential at 25oC is + 0.199 V (vs) SHE while the potentials with 1.0 M and 0.1 M KCl are +0.237 and + 0.290 V (vs) SHE, respectively.
This electrode can be easily constructed and is as shown in Figure. The electrode is contained in a glass tube fitted with a 10 mm fritted glass disk or porous plug (A). A layer of agar-KCl gel (B) is placed on the top of the disk to prevent loss of solution from the half-cell. A portion of the hot suspension is poured into the tube, which on cooling solidified into a gel with a low electric resistance. A layer of solid KCl (C) is placed on the gel and the tube is filled with saturated KCl (D). A drop or two of 0.1 M Ag NO3 is added and a heavy gauge Ag wire (E) is inserted in the solution.
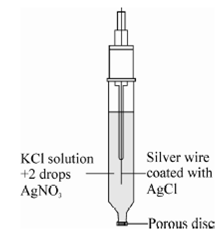
Figure: A typical silver-silver chloride reference elctrode