laboratory-made saturated calomel reference electrodes:
Calomel electrodes may be fabricated in a variety of sizes and shapes, some of these are shown in Figure. Some calomel electrodes are small enough to be inserted into blood vessels of experimental animals through syringe needles. It should be emphasized that the potential of an electrode does not depend upon its size. Likewise, the liquid junction between the test solution and the KCl solution in the electrode may be physically established in various ways. Sometimes it involves an agar plug or a sintered glass frit, sometimes a pinhole, sometimes a small wet fiber, the point is to permit the migration of ions without allowing excessive solution to pour across the interface. Although the contamination from calomel electrodes is inconsequential for most purposes, in critical cases it must be remembered that a small amount of KCl may leak into the test solution. Similarly, a test solution with a large hydrostatic head may contaminate the contents of a calomel electrode.
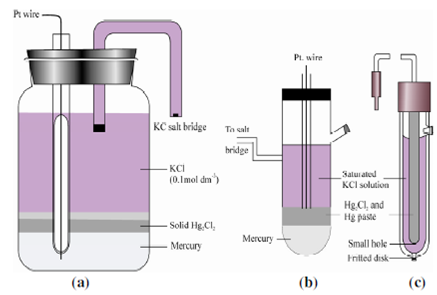
Figure: A typical (a) & (b) laboratory-made saturated calomel reference electrodes; (c) commercial calomel electrode
For special purpose, modification of the calomel electrode may be preferred. Thus, if it is necessary to avoid the presence of potassium ions, the electrode may be prepared with sodium chloride replacing the potassium chloride. In some cases, the presence of chloride ions may be inimical and a mercury (I) sulphate electrode may then be used, this is prepared in a similar manner to a calomel electrode using mercury (I) sulphate and potassium or sodium sulphate solution.