Calomel Electrode
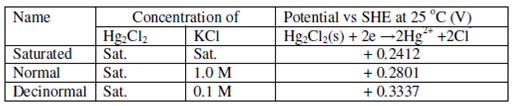
The calomel electrode is the most widely used electrode due to its ease of preparation and constancy of potential. A calomel half-cell is one in that mercury and calomel [mercury (I) chloride] is covered with aqueous KCl solution of definite concentration (this may be 0.1M, 1.0 M, or saturated). The electrode is called the saturated calomel electrode If the KCl solution is saturated with the calomel. The electrode potential of such electrode is +0.2412 V at 25ºC as determined against SHE. If the KCl solution is exactly 1.0 M, the potential is +0.2801 V; this is sometimes called Standard Calomel Electrode (SCE). The Electrode potential of various category of calomel electrodes are given in Table 2.1.
Table 2.1: Specifications of Calomel Electrodes
The half-cell reaction for this electrode is:
Hg22++ 2e → 2 Hg ... (2.24)
It may be recalled that mercury (I) is a dimeric species, i.e. we are dealing with Hg(I) but ion in the solution is Hg22+ . According to Nernst equation, the potential is given by
E = Eº - (0.0591/2 )log 1/[ Hg22+] ... (2.25)
Hg22+ is also involved in another equilibrium : Hg2Cl2 is a slightly soluble salt, for which we may write a solubility product constant, Ksp.
Ksp = [Hg22+][Cl-]2 ... (2.26)
Rearranging, we obtain
[Hg22+] = Ksp/[Cl-]2 ... (2.27)
and substitution into the Nernst equation above yields
E= Eº - (0.059/2 )log[Cl-]2/ Ksp ... (2.28)
Since E0 and Ksp are constants, it is seen that the potential reflects the concentration of Cl¯ .