Residual Current:
Even within the absence of electroactive substance in the solution having only supporting electrolytes a small current is always present which is referred as residual current which consists of two components.
The first is reduction of trace impurities (faradaic current) present in the large concentration of supporting electrolyte used. These involve dissolved oxygen, traces of metal ions such as ferric iron, lead and arsenic. Out of those, dissolved oxygen could be removed through passing pure nitrogen for 10 to 15 minutes before taking C-V curve.
Another source is charging current or capacitive current that is non-faradaic and is present while the working electrode potential changes. Mercury is unique within remaining electrically uncharged while it is dropping freely into the supporting electrolyte. A small current which is observed from supporting electrolyte solution is because of continuous charging of new mercury drop to the applied potential in order to remain neutral. The mercury is covered along with an electrical double layer of negatively and positively charged ions of the electrolyte. The capacity of the double layer and therefore the charging current vary depending on the potential that is imposed upon mercury.
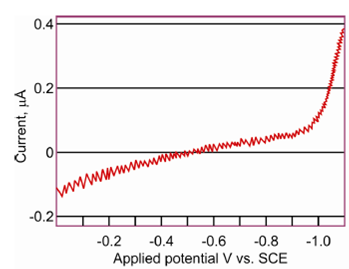
Figure: Residual current for a 0.1 M solution of HCL
A residual current is responsible for determining the accuracy and sensitivity of a polarographic method at low concentrations of electroactive substances below 10-4 M. At concentrations around 10-5M of electroactive species the residual current is larger than the faradaic current of the substance to be determined and therefore not sensitive.
A residual current has to be compensated at the potential at that the diffusion current is measured.