Interpretation of DTA Curve:
DTA curves of a pure compound represent features of in which compound for physical chemical changes. By using DTA curve one could co-relate the changes within energy since of thermophysical and chemical change occurring in a compound since of heating the material. This can frequent gives us directly to the temperature at that the physico- chemical transition are occurring and that is also used to identify the presence of respective elements or compounds qualitatively. The modifications in DTA curve additional gives information about the thermophysical changes related along with mass change e.g. melting point, glass transition temperature, crystallization temperature etc.
To further described, let's consider the instance of CaC2O4.H2O for that DTA curve is display in Figure. This curve denotes in which out of three DTA peaks first is endothermic in nature, second is exothermic and third one is again endothermic within nature. The correlates the TGA output and confirms in which the nature of reactions occurring within the endothermic are since of desolvation and decarboxylation although exothermic is because of decomposition followed through oxidation and finally formation of stable oxide CaO along with evolution of carbon dioxide gas. This could be explained through the chemistry of decomposition of CaC2O4.H2O while it is heated.
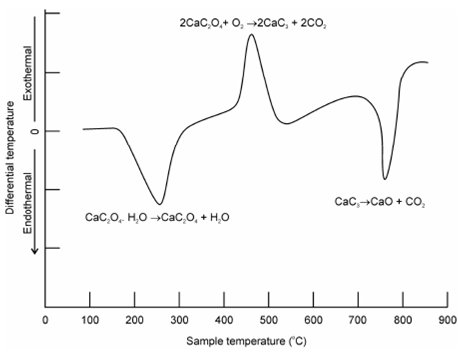
Figure: DTA curve of CaC2O4.H2O in the presence of O2