Diazonium coupling:
One time the diazonium salt has been formed, it can be treated with several nucleophiles like Br, Cl, I, CN and OH (Fig. 12). The nucleophile displaces nitrogen from the aromatic ring and the nitrogen that is formed is lost from the reaction mixture as a gas, so helping to drive the reaction to completion.
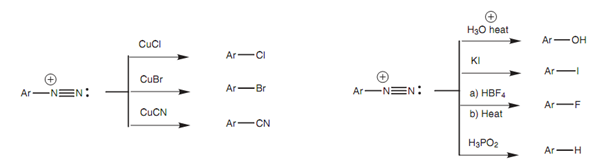
Figure: Reactions of diazonium salts.
Those reactions including Cu (I) are also termed as the Sandmeyer reaction. Diazonium salts are also employed in a reaction called diazonium coupling in which the diazonium salt is coupled to the para position of a phenol or an arylamine.
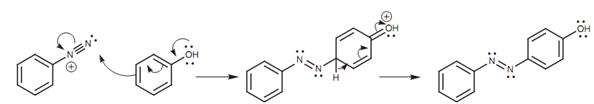
Figure: Diazonium coupling.
The azo products acquired have an extended conjugated system that involves both aromatic rings and the N=N link. As a result, these compounds are frequently colored and are employed as dyes.
The above coupling is much more proficient if the reaction is performed under slightly alkaline conditions (NaOH) such that the phenol is ionized to a phenoxide ion (ArO). Phenoxide ions are much more reactive to electrophilic addition as compared to phenols themselves. Strong alkaline conditions cannot be employed because the hydroxide ion adds to the diazonium salt and avoids coupling. If the para position of the phenol is already occupied, diazo coupling can happen at the ortho position instead.
Aliphatic amines, also secondary and tertiary aromatic amines, react with nitrous acid, but these reactions are less helpful in organic synthesis.