NMR spectrum of diphenylethene:
An aldehyde proton as well experiences a secondary magnetic field because of diamagnetic circulation, but in addition there is an inductive effect resultant from the electron withdrawing effect of the carbonyl group. So, an aldehyde proton experiences two deshielding effects, which means that it has a higher chemical shift as compared to even an aromatic ring. Usually, the signal appears approximately 9.6 ppm. The combined effects of diamagnetic circulation and inductive effects as well result in high chemical shifts for the OH of a carboxylic acid in which the signal can have a chemical shift larger than 10 ppm.
For several unsaturated systems, diamagnetic circulation sets up a secondary field that improves the applied magnetic field. The exception is alkynes in which the secondary field opposes the applied field and causes shielding.
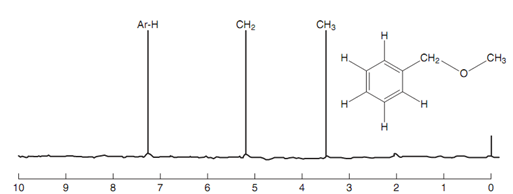
Figure: NMR spectrum of 1, 1-diphenylethene.