Hydrogen Ion Concentration:
There are a number of substances whose ionic state in solution is greatly influenced by the presence of hydrogen ions. For instance, the aqueous solution of potassium dichromate includes the chromate ion-dichromate ion equilibrium as shown below:
2CrO72- + 2H + ↔ Cr2O72- + H2O
Chromate ion Dichromate ion
( λ max,375 nm) ( λ max 350,450 nm)
The chromate ion has a single λmax at 375 nm whereas dichromate ion has two peaks in the spectra; λmax at 350 and 450 nm. The location of equilibrium depends on the pH of the solution and yellow color of solution changes to orange on increasing the concentration of hydrogen ions. Thus, the results of the determination of chromate ion ( CrO72-) concentration will depend on the pH. Therefore, it is imperative in which substances, whose color is influenced through change within hydrogen ion concentration, must be studied under the condition of similar pH.
In a few cases, two absorbing species are in equilibrium and have a general value of absorptivity at a certain wavelength. For instance, in case of bromothymol blue the absorption spectra at different pH values are different. Thus, at wavelength of 501 nm, we see which all species have similar molar absorptivity. Thus, no matter to what extent does one species change within the other, there is no change in the total absorption. Like a wavelength is known as isosbestic point. At this wavelength the Beer's law holds, by the measurements have low sensitivity. Therefore, such wavelengths should be prevented for the quantitative work.
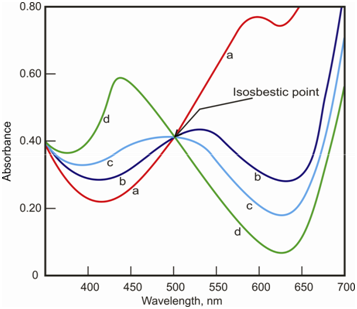
Figure: The absorption spectra for bromothymol blue at different pH values showing the isosbestic point at 501 nm