Identification of organic compounds:
Now-a-days, the thin layer plates and sheets incorporating fluorescent dyes in powdered adsorbent are commercially available. When these are held under ultraviolet radiation, dark spots glow while sample spots occur because of fluorescent substances. Alternatively, an immobile fluorescent substance may be added while preparing the slurry so that the separated compounds appear as dark spots against fluorescing background when the plate is viewed under the ultraviolet light.
Another common technique used for the identification of organic compounds is by spraying the plate with concentrated sulphuric acid solution and then heating it in an oven. Charring of the organic compounds make them appear as black spots.
The Rf values in TLC are difficult to reproduce because of experimental variables. Following factors may be considered.
i) Nature of the adsorbent-its chemical nature, particle size, surface area and binder. Also its activity, thickness and uniformity.
ii) Nature of mobile phase-its purity, moisture content, precision of mixing in case of mixture and volatility.
iii) Amount of sample used
iv) Vapour-pressure equilibrium among the plate and the atmosphere of the development chamber
v) Ambient temperature
A classical thin layer chromatogram of a mixture is shown in Figure.
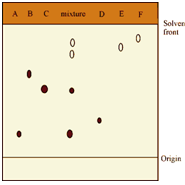
Figure: Thin layer chromatogram of a mixture
The chromatograms can be stored for future reference or their photographs can be made.