Halocarbon Compounds:
These compounds are represented by a three digit nomenclature. Here, the first digit shows the number of carbon atoms in the compound minus one, the second digit represent for the number of hydrogen atoms plus one whenever the third digit refers for the number of fluorine atoms. The remaining atoms are chlorine.
As an instance, let us assume the refrigerant having R22 as its three digit nomenclature.
According to the above indicated convention,
No. of C atoms in R22: C - 1 = 0 => C = 1
No. of H atoms in R22: H + 1 = 2 => H = 1
No. of F atoms in R22: F = 2
As there is just one carbon atom in the compound, this has originated from the methane series (CH4). From the estimation, we may see there are one hydrogen atom and two fluorine atoms. The remaining valence bond of carbon shall be balanced through chlorine. Therefore, the substance is
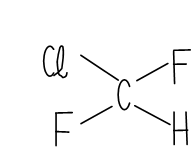
Graphical Representation of Monochloro-Difluoro-Methane
Thus, chemical formula of R22 is CHClF2 and contain the name Monochloro-difluoro-methane .
Again taking the example of R134, we can determine its chemical formula as above which gives us as following
No. of C atoms: C - 1 = 1 => C = 2
No. of H atoms: H + 1 = 3 => H = 2
No. of F atoms: F = 4
Thus, no. of Cl atoms: Cl = 0
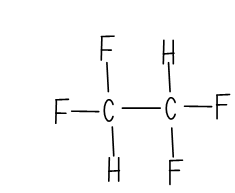
Graphical Representation of Tetrafluoroethane
The compound is C2H2F2 and it's called as Tetrafluoroethane .The non-halogenated refrigerants follow a different naming convention which is based upon the series of the refrigerant.