Elution of Columns:
After having learnt the classification of chromatographic methods, it is important to look into some concepts which may explain the separation process. From the discussion, it can be noted that the separations are conducted on columns or on planar surfaces. The equilibria upon which the two types of chromatography are based are identical. The theory developed for column chromatography is adapted to planar chromatography.
To start with we look into a simple picture as to how the two substances X and Y are separated on a column by elution with a liquid as a mobile phase. Actually, elution involves washing a species through a column by continuous addition of fresh solvent. The different stages of separation are schematically shown in Figure. The sample is introduced at the head of column (time t0) where the components of the sample distribute themselves between the two phases. Further accumulation of mobile phase pushes the solvent containing a part of the sample down the column. Here further partition between the mobile phase and fresh portion of stationary phase occurs (time t1). Simultaneously, partitioning between the fresh solvent and the stationary phase is taking place at the site of the original sample.
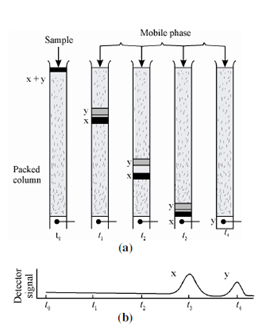
Figure: (a) Column separation of a mixture of components X and Y; and (b) Output of signal detector at various stages