Bromination of nitrobenzene:
Deactivating groups form electrophilic substitution more hard but the reaction will carry on within more forcing reaction conditions. Though, substitution is now directed to the meta position. This can be described through comparing all the possible resonance structures taking place from ortho, Meta and para attack. Like an instance, we shall consider the bromination of nitrotoluene.
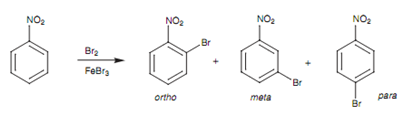
Figure: Bromination of nitrobenzene.
Of all the feasible resonance structures taking place from ortho, meta, and para attack, there are two particular resonance structures (occurring from ortho and para attack) in which the positive charge is located directly next to the electron-withdrawing nitro group. The result of it is that, these resonance structures are greatly destabilized. This does not take place with any of the resonance structures arising from meta attack and thus meta attack is favored.
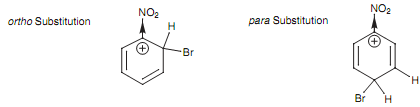
Figure: Destabilizing resonance structures for the intermediate arising from ortho and para substitution.