Crucial resonance structures:
There are 3 resonance structures for each of the three intermediates directing to these products, but the crucial ones to consider are those that position a positive charge next to the substituent. These take place with ortho and para substitution, but not meta substitution.
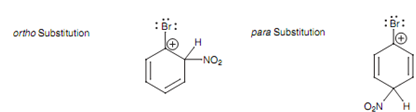
Figure: Crucial resonance structures for ortho and para substitution.
These are the vital resonance structures as far as the directing properties of the substituent are referred. If bromine works inductively, it will destabilize these intermediates and direct substitution to the Meta position.
Though, we make out that bromine directs ortho/para and thus it must be stabilizing the ortho/para intermediates than destabilizing them. The one way that bromine can stabilize the neighboring positive charge is through resonance in similar way like a nitrogen or oxygen atom. So, the bromine works as a nucleophile and donates one of its lone pairs to form a new bond to the electrophilic center beside it. A new π bond is created and the positive charge is moved onto the bromine atom. This resonance effect is weak because the halogen atom is a much weaker nucleophile as compared to the oxygen or nitrogen and is less able of stabilizing a positive charge. Though, it is important enough to direct substitution to the ortho and para positions.
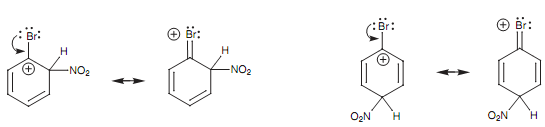
Figure: Resonance interactions involving bromine.
For halogen substituents, the inductive effect is much more significant than the resonance effect in deactivating the ring. Though, once electrophilic substitution does occur, resonance effects are more significant than inductive effects in directing substitution.