Dynein
It is a very large protein (1000-2000 kDa)which consisting of one, two or three heads based on the source. Such as the heads of myosin and the heads of dynein form cross-bridges, in this case having the B subfibers, and possess ATPase activity. Binding of ATP to dynein causes it to dissociate from the B subfiber. The dynein binds again with the B subfiber on hydrolysis of the ATP to ADP and Pi, having the subsequent release of the Pi and ADP (a cycle very same to that which take place with the binding of the S1 heads of myosin to ATP). This particular ATPase cycle leads to the movement of the cilium as the outer doublets of the axoneme slide past each other. The force between adjacent doublets is generated by the dynein
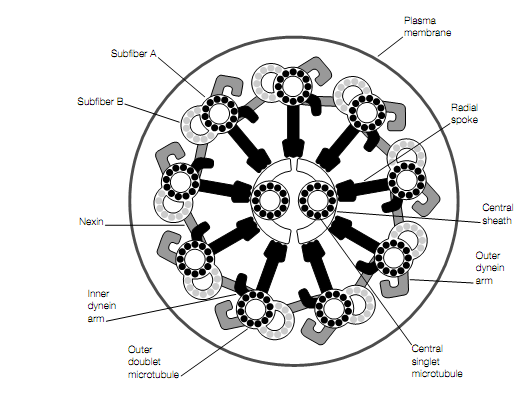
Cross-sectional diagram of a cilium.
cross-bridges. Therefore, the dynein arms on subfiber A of one doublet walk along subfiber B of the adjacent doublet. Unlike in muscle, in a cilium where the actin and myosin filaments slide past each other the radial spokes resist the sliding motion, which instead of it converted into a local bending. The extremely extensible protein named nexin, keeps adjacent doublets together during this process.
A defect or absence in any one of the proteins within the axoneme (for example : nexin, dynein etc.) results in cilia that are immotile, so called as immotile-cilia syndrome. Patients who suffering from this disease due to the cilia have chronic pulmonary disorders in the respiratory tract being unable to sweep out bacteria and other foreign particles. As well males with this genetic defect are infertile because due to flagella inactivity their sperm are unable to move.