Newman projections of the chair conformations of methylcyclohexane:
This can be demonstrated by comparing Newman diagrams of the two methylcyclohexane conformations shown in the below figure.
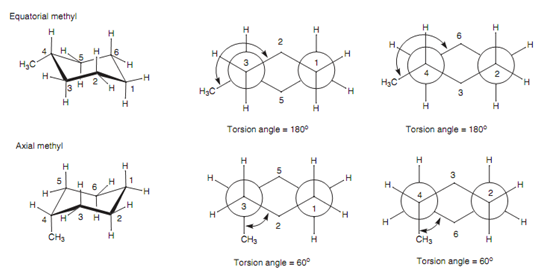
Figure: Newman projections of the chair conformations of methylcyclohexane.
A torsion angle of 60? among the C-C bonds represents a gauche interaction and thus an axial methyl substituent experiences 2 gauche interactions along with the cyclohexane ring while the equatorial methyl substituent experiences none. The result of it is, the latter chair conformation is chosen and about 95% of methylcyclohexane molecules are in this conformation at any one time, as compared to 5% in another conformation.