Equatorial C–H bonds:
Though, in the boat conformation, bond 1-2 is eclipsed along with bond 3-4, and bond 1-6 is eclipsed along with bond 5-4. The meaning of this is that the boat conformation is less stable as compared to the chair conformation and the vast majority of cyclohexane molecules available in the chair conformation. Though, the energy barrier is sufficiently small for the cyclohexane molecules to pass by the boat conformation in a process termed as 'ring flipping. The capability of a cyclohexane molecule to ring- flip is significant while substituents are exists. Each carbon atom in the chair structure comprises two C-H bonds, but these are not similar. One of these bonds is known as equatorial because it is roughly in the plane of the ring. Another C-H bond is vertical to the plane of the ring and is termed as the axial bond.
While ring flipping occurs from one chair to another, all the axial bonds become equatorial bonds and all the equatorial bonds turn into axial bonds. This does not subject for cyclohexane itself, but it becomes significant while there is a substituent exist in the ring.
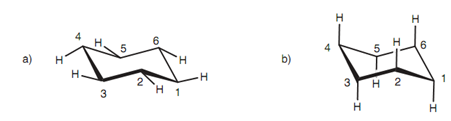
Figure: (a) Equatorial C-H bonds; (b) axial C-H bonds.