Claisen–Schmidt reaction:
This reaction can be limited through only comprising benzaldehyde and sodium hydroxide basically present in the reaction flask. Because benzaldehyde has no α-protons, no reaction can occurs. Now a small quantity of ethanal can be added. Reaction with excess sodium hydroxide turns most of the ethanal into its enolate ion. There will just only be an extremely small amount of 'free' ethanal left as compared to benzaldehyde and thus the enolate ion is more probable to react with benzaldehyde.
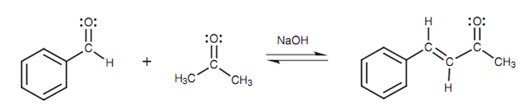
Figure: Claisen-Schmidt reaction.
One time the reaction is judged to have occurred, the next small addition of ethanal can happen and the process is repeated again.
Ketones and aldehydes can as well be related by similar method - a reaction termed as the Claisen-Schmidt reaction. The most successful reactions are those in which the aldehyde does not contain an α-proton.