Van der Waals Forces:
Further to chemical bonding among atoms, there is another category of attractive force which exists among ions, atoms, or molecules known as van der Waals forces.
These forces occur among the molecules of nonpolar covalent substances such as H2, Cl2, and He. Those forces are commonly believed to be caused through a temporary dipole or unequal charge distribution, since electrons constantly move about within an ion, atom, or molecule. At a given example, more electrons might be in one region than in another region, as described in Figure.
The temporary dipole induces a same temporary dipole on a nearby atom, ion, or molecule. Each instant, billions of these temporary dipoles form, break apart and regenerate to act as a weak electrostatic force of attraction called as van der Waals forces.
It is significant to remember that van der Waals forces exist among all types of molecules. A few molecules might have these forces as well as dipole or other intermolecular forces. Van der Waals forces, therefore, are the just intermolecular bonds among nonpolar covalent molecules like as H2, Cl2, and CH4. The number of electrons in a substance increases as the gram molecular mass (mass in grams of one mole of compound) rises. Thus, the strength of the van der Waals forces among substances rises along with increasing gram molecular mass.
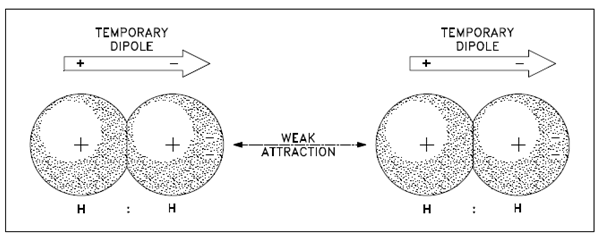
Figure: Van der Waals Forces
Van der Waals forces are little compared to the forces of chemical bonding and are important only while the molecules are extremely close together.