Silver coulometer:
The silver coulometer is an instance example of a gravimetric coulometer in that the amount of metal deposited at cathode or the amount of metal stripped from an anode is denote.
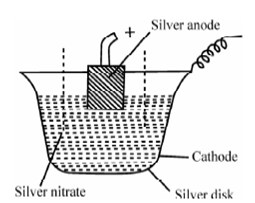
Figure: Silver Coulometer
A silver coulometer is display in Figure is the most satisfactory either in the cathodic deposition mode or better since, in the anodic stripping mode in perchloric acid media.
A convenient and easy form of silver coulometer consists of a platinum disk or silver disk that acts as a cathode and contains a solution of 1 M silver nitrate as the electrolyte. The rod of pure silver enclosed in a porous pot acts as the anode (l coulomb of electricity corresponds to around l mg of silver and this should be considered along with reference to electrode accuracy, size of weighing and sample size.). A current density at the anode should not exceed 0.2 A cm-2. After electrolysis, the cathode is dried weighed. The rise in mass of the cathode provides the amount of silver deposited. To the mass of the silver deposited, the coulomb included in the reaction could be compute.