Cardiovascular regulation
Long-term regulation of blood pressure does not need the ANS and relies on control of blood volume and osmolality through vasopressin and the renin–angiotensin–aldosterone cascade. Though, the ANS is vital for the short-term regulation of mean arterial blood pressure (MAP). At rest the ANS activates to maintain a roughly steady MAP by negative feedback. Mean arterial pressure is the result of cardiac output (Q) that is the volume output of the left ventricle per minute, and the peripheral resistance (R), that is associated to the radius of the arterioles.
Now, the cardiac output is in return a product of stroke volume, the volume expelled from the left ventricle per beat, established by the contractile force of the heart, and heart rate. Therefore cardiac output can be increased (or lowered) either by increasing (or decreasing) stroke volume or heart rate or both. The ANS regulates cardiac output through both sympathetic and parasympathetic supply to the heart. Both are tonically active at rest and raises (reduces) in cardiac output are achieved by raising (or lowering) sympathetic activity and decreasing (elevating) parasympathetic activity. In humans the sympathetic activity raisesboth force and rate, while parasympathetic activity lowers rate, though has little effect on force as few parasympathetic fibers innervate the ventricles.
Peripheral resistance is controlled exclusively by modifying the tonic firing rates of sympathetic neurons going to vascular smooth muscle. Raised firing frequency causes vasoconstriction that raises peripheral resistance.
The circuitry for negative feedback regulation of MAP exists in the medulla as shown in figure below. Baroreceptors are stretch receptors situated in the carotid sinus and the aortic arch whichare sensitive to fast modifications in MAP. Their afferents run in the glossopharyngeal (IX) and vagus (X) cranial nerves correspondingly and finish in the nucleus of the solitary tract (NST), a structure included in a broad variety of visceral reflexes (example, swallowing, and chemoreceptor responses). The NST plans to the dorsal vagal nucleus (DNX) and to the nucleus ambiguus (NA), both of which give increase to preganglionic parasympathetic axons which run in the vagus nerve to the heart. The NTS controls sympathetic outflow to heart and blood vessels by input to the caudal ventrolateral medulla (CVLM). This consists of GABAergic inhibitory neurons that synapse in the rostral ventrolateral medulla, axons of that run down the spinal cord, finishing on preganglionic sympathetic neurons. A increase in MAP raises the firing rate of baroreceptor afferents, and this straightforwardly activates the parasympathetic innervation to the heart, slowing its rate. Though, the presence of inhibitory neurons in the CVLM means that baroreceptor release suppresses sympathetic outflow to the heart, reduces its rate and force of contraction, and arterioles that reduces peripheral resistance. The total effect is a fall in blood pressure back to the set point. Responses take place in the reverse direction to an initial drop in MAP.
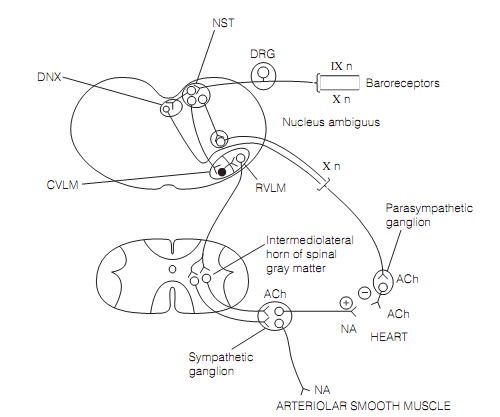
Figure: Brainstem circuits controlling mean arterial blood pressure. Here, DRG=dorsal root ganglion; DNX=dorsal vagal nucleus; CVLM=caudal ventrolateral medulla; RVLM=rostral ventrolateral medulla; and NST= nucleus of the solitary tract.
Cardiovascular variables are altered to match situations. During exercise the cerebellar and cerebral cortex act to alter hypothalamic autonomic regulation. Likewise cardiovascular responses seen in emotional states need limbic system components like the amygdala and the cingulate cortex.