Hormonal control by epinephrine and glucagon:
Glycogen metabolism is strongly controlled through hormones. When blood glucose levels fall, glucagon is secreted through the cells of the pancreas and acts on the liver to stimulate glycogen breakdown to glucose that is then released into the bloodstream to boost blood glucose levels again. Nervous stimulation or Muscular contraction (the 'fight or flight' response) causes the release of epinephrine (adrenaline) from the adrenal medulla and these acts on muscle to raise glycogen breakdown ready to supply the energy required of the cells.
Consider first the activation of glycogen degradation through epinephrine in the liver. The hormone binds to a receptor, known as the β-adrenergic receptor, in the plasma membrane of the goal cell shown in the figure. Binding of the hormone to the receptor causes a conformational modification in the protein that in turn activates an enzyme known as adenylate cyclase. The receptor does not activate adenylate cyclase straightly but rather through activating a G-protein as an intermediary in the signaling process. Activated adenylate cyclase converts ATP to 3'5' cyclic AMP and cAMP. The cAMP connects to protein kinase A or PKA that is also known as cAMP-dependent protein kinase. That enzyme consists of two regulatory subunits that are (R) and two catalytic subunits (C), making a complex, R2C2 which is generally inactive. The binding of two molecules of cAMP to every of the regulatory subunits leads to dissociation of the difficult into an R2 complex and two free C subunits that is now catalytically active. The active PKA phosphorylates phosphorylase kinase that can exist as an inactive dephosphorylated form and an active phosphorylated form. Therefore phosphorylase kinase is now also activated and in turn phosphorylates phosphorylase b, translating it to the active phosphorylase a now carries out a rapid degradation of glycogen.
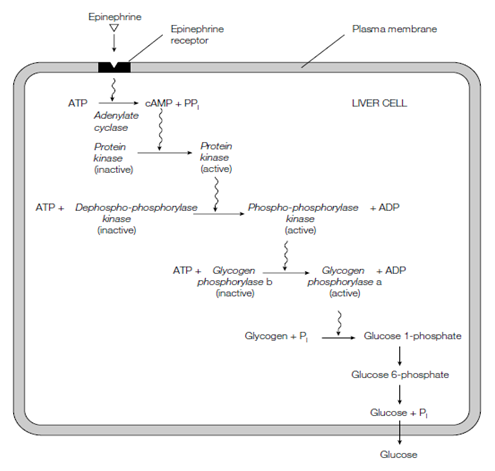
Figure Mechanism of action of epinephrine.
This set of reactions is known as a cascade and is organized so as greatly to amplify the original signal of a small number of hormone molecules. For instance, each bound hormone causes the production of several cAMP molecules inside the cell; the activated protein kinase A in turn activates various molecules of phosphorylase kinase; each active phosphorylase kinase activates several molecules of phosphorylase. Therefore a small hormonal signal can cause a main shift in cell metabolism.
To avoid the operation of a futile cycle, it is necessary that glycogen synthesis is triggered off during epinephrine or glucagon stimulation of glycogen breakdown. This is achieved through the activated protein kinase A which, as well as phosphorylating phosphorylase kinase also phosphorylates glycogen synthase A, translate it to the inactive synthase b form. Therefore protein kinase A activates glycogen degradation and inhibits glycogen synthesis.
When epinephrine and glucagon levels in the bloodstream fall over, the hormone dissociates from the receptor, no more cAMP is build and existing cAMP is converted to 5' AMP instance example 'normal' AMP not the cyclic form through cAMP phosphodiesterase, an enzyme which is constantly active in the cell. This refuse
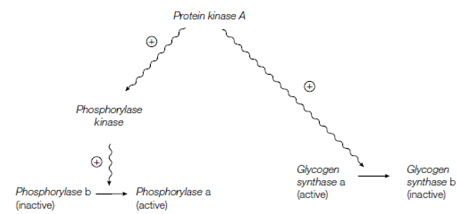
Figure: Dual control of glycogen degradation and glycogen synthesis by protein kinase A.
in the cAMP level shuts off the activation cascade. The enzymes which had been phosphorylated are now dephosphorylated through protein phosphatase I, restoring them to their real condition.