Central regulation of feeding and satiety
Food intake is regulated in the CNS by two parallel brain pathways which originate in the arcuate nucleus of the hypothalamus. An orexigenic pathway promotes feeding and reduces energy expenditure whilst an anorexigenic pathway reduces feeding and increases energy expenditure in Figure. Both exert their effects by modulating the nucleus of the solitary tract (NST). This structure organizes feeding reflexes and regulates energy expenditure by altering sympathetic nervous system output, basal metabolic rate, and locomotor activity.
Arcuate nucleus NPY or AgRP neurons, so called because they use neuropeptide Y and agouti-related protein as co-transmitters are the first-order neurons in the orexigenic pathway. NPY secretion from the NPY or AgRP neurons is stimulated by ghrelin. NPY/AgRP neurons project to the lateral hypothalamus and synapsing with second-order neurons which use peptides termed orexins as neurotransmitters. These relay to neurons in the nucleus of the solitary tract (NST). NPY/AgRP cells also produce GABA and inhibit the anorexigenic pathway.
First-order cells in the anorexigenic pathway in the arcuate nucleus contain pro- piomelanocortin (POMC), the precursor protein for several biologically active peptides, one of which, melanocortin, is a neurotransmitter of these neurons.
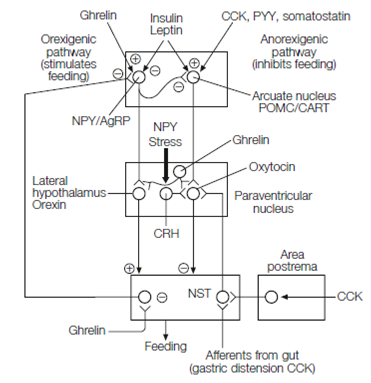
Figure: Central pathways controlling feeding. CRH, corticotrophin releasing hormone; POMC, proopiomelanocortin neuron (releases melanocortin); NPY, neuropeptide Y; AgRP, agouti-related protein; CART, cocaine- and amphetamine-related transcript; NST, nucleus of the solitary tract; CCK, cholecystokinin.
Hence the anorexigenic pathway is often described as the melanocortin system. Other first- order cells in the anorexigenic pathway express cocaine- and amphetamine-related transcript (CART). POMC/CART cell axons run to the paraventricular nucleus (PVN) of the hypothalamus which contains neurons which use oxytocin, thyrotrophin producing hormone or corticotrophin releasing hormone as transmitters. The melanocortin system gets a wealth of inputs relaying short- and long-term satiety signals, and also possibly from a glucostat in the arcuate nucleus. Activation of the melanocortin system inhibits feeding and increases basal metabolic rate via outputs from the NST.Leptin and insulin receptors exist on first-order neurons of both orexigenic and anorexigenic pathways. Leptin and insulin inhibit the orexigenic pathway and stimulate the anorexigenic pathway, thereby reducing feeding.
Stress and neuropeptide Y stimulate CRH neurons in the PVN which synapse with melanocortin system neurons to inhibit feeding. Stress is a key component of anorexia nervosa in which patients self-starve. Brain imaging shows that anorexics produce amygdala fear responses to their own body image. Once established, starvation naturally maintains high activity in the hypothalamic pituitary stress axis.
Endocannabinoid pathways are activated in fasting and stimulate feeding through modulating the action of leptin on the melanocortin system, and by stimulating the mesolimbic reward system.