Mercury cathode:
Mercury cathode is particularly meaningful for removing simply decreasing metals as a preliminary step in an analysis. For instance, nickel, silver, copper, cobalt, and cadmium are readily separated at this electrode from ions, like as aluminium, titanium, the alkali metals, phosphates and sulphates. The precipitated elements dissolve in mercury along with little hydrogen evolution since mercury has a high hydrogen overvoltage and therefore no hydrogen evolution even at high applied potentials.
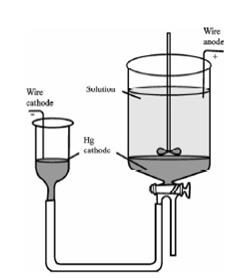
Figure: A mercury cathode for the electrolytic removal of metal ions from solution
Benefits of mercury cathode
1. It forms an amalgam along with a number of metals
2. It has a high hydrogen overvoltage and therefore no hydrogen evolution even at high potentials.