Electrophilic addition:
The conjugated diene's reactions reflect the fact that a conjugated diene should be observed like a functional group in its own right, rather than like two separate alkenes. Electrophilic addition to a conjugated diene results in a mixture of two feasible products that are arising from 1, 2-addition and 1, 4 addition displayed in figure.
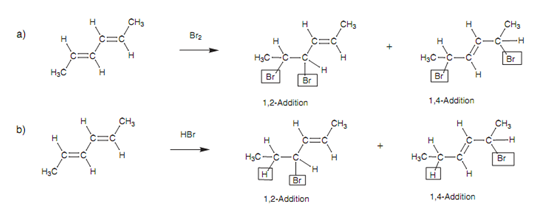
Figure: Electrophilic addition to a conjugated diene of (a) bromine and (b) HBr.
In 1, 2-addition, new atoms have been added to every end of one of the alkene units. This is the general electrophilic addition of an alkene along with which we are known. In 1, 4-addition, new atoms have been added to each end of the complete diene system. Additionally, the double bond remaining has shifted position (isomerized) to the 2, 3 position.