Diels-Alder cycloaddition:
The Diels-Alder cycloaddition reaction is a significant method through which six-membered rings can be synthesized. The reaction includes a conjugated diene and an alkene as displayed in figure.
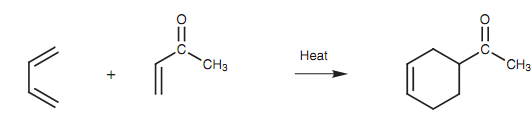
Figure: Diels-Alder cycloaddition.
The alkene is considered to like a dienophile (diene-lover) and generally has an electron-withdrawing group related to it (for example a carbonyl group or a nitrile). The mechanism is planned with new bonds being created at the same time as old bonds are being broken. No intermediates are included.

Figure: Mechanism of Diels-Alder cycloaddition.