Bonding:
A conjugated diene does not act like two isolated alkenes. For instance, the length of the 'single' bond connecting the two alkene units is little shorter as compared to one would suppose for a typical single bond (1.48 Å vs. 1.54 Å). This illustrates that there is a certain amount of double-bond character present in this bond. Two descriptions can be employed to account for this.
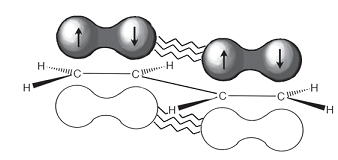
Figure: π-Orbital overlap.
Very firstly, the bond in question connects two sp2 hybridized carbons than two sp3 hybridized carbons. Hence, a sp2 hybridized orbital from each carbon is employed for the single bond. Because this hybridized orbital has more s-character as compared to a sp3 hybridized orbital, the bond is supposed to be shorter. An alternative description is that the π orbitals of the two alkene systems can overlap to generate the partial double-bond character.