Alkanes:
Conformational isomers take place from the rotation of C-C single bonds. There are several different shapes that a molecule such as ethane could adopt by rotation around the C-C bond.
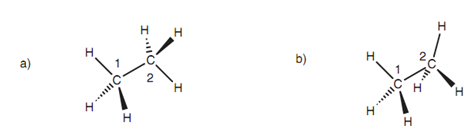
Figure: (a) 'Staggered' conformation of ethane ; (b) 'eclipsed' conformation of ethane
Though, it is helpful to concentrate on the most distinctive ones. The two conformations I and II are termed as 'staggered' and 'eclipsed' correspondingly. In conformation I, the C-H bonds on carbon 1 are staggered along with respect to the C-H bonds on carbon 2. In conformation II, they are eclipsed. Newman projections present the view together the C1-C2 bond and emphasize the variation. Carbon 1 is presented through the small black circle and carbon 2 is presented through the larger sphere.