Alkenes - Z and E nomenclature:
For the alkenes cis and Trans nomenclature is an old method of classifying the configurational isomers of alkenes and is still usually employed. Though, it is only appropriate for simple 1, 2-disubstituted alkenes in which one can compare the relative position of the 2 substituents along with respect to each other. While it comes to trisubstituted and tetrasubstituted alkenes, a dissimilar nomenclature is needed.
The Z/E nomenclature permits a clear, clear definition of the con?guration of alkenes. The method via which alkenes are classified as Z or E is demonstrated in below diagram. First of all, the atoms directly linked to the double bond are recognized and given their atomic number. After that in the next stage is to compare the 2 atoms at each end of the alkene. The one that has the highest atomic number takes priority on the other. At the left side, oxygen has a higher atomic number as compared to the hydrogen and takes priority. At the right side, both atoms are similar (carbon) and we cannot choose among them.
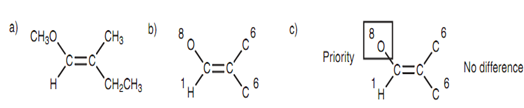
Figure: (a) Alkene; (b) atomic numbers; (c) priority groups.