Alkenes - cis and trans isomerism:
Alkenes having similar substituents at either end of the double bond can just only exist as one molecule. Though, alkenes comprising dissimilar substituents at both ends of the double bond can exist like two possible isomers. For instance, 1-butene has 2 hydrogens at one end of the double bond and there is just only one way of constructing it. Alternatively, 2-butene has dissimilar substituents at both ends of the double bond (H and CH3) and can be constructed in two manners. The methyl groups can be on similar side of the double bond (the cis isomer), or on opposite sides (the Trans isomer). The alkenes' cis and Trans isomers are Configurational isomers (also termed as geometric isomers) since they have different shapes and cannot interconvert because the double bond of an alkene cannot rotate. Hence, the substituents are 'fixed' in space relative to each other. The structures are different compounds along with different chemical and physical properties.
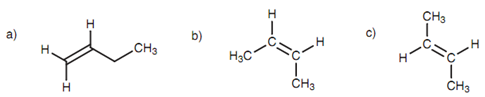
Figure: (a) 1-Butene; (b) cis-2-butene; (c) trans-2-butene.