Electrolytic Conductance:
An electrolytic solution contains free ions in addition to other kinetically identifiable species. When electrical potential is applied across the solution the macroscopic observations are, the flow of current through the solution and the chemical changes generally resulting in the liberation or dissolution of the electrode material at the points where the current enters or leaves the solution. This phenomenon of decomposition of the solutions by electrical current is termed as electrolysis.
Electrolytic conduction, in that charges carried through ions, will not occur unless the ions of the electrolyte are free to move. Therefore, electrolytic conduction is exhibited principally by molten salts and by aqueous solutions of electrolytes. A principle of electrolytic conduction is best illustrated by reference to an electrolytic cell such as that shown in Figure for the electrolysis of molten NaCl between inert electrodes. The entire assembly except that of the external battery of Figure is known as the cell.
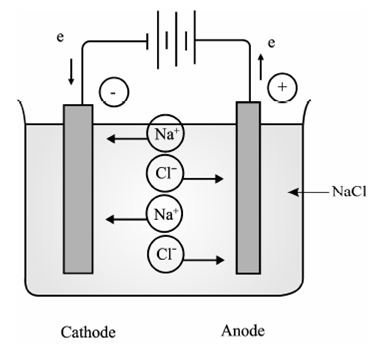
Na++e→ Na Cl-→1/2 Cl2 +e
Figure: Electrolysis of molten sodium chloride