Theoretical Conductivity as a Function of pH:
In both Figures, a definite relationship exists among pH and conductivity, supposing no foreign ions is present. A same graph could be constructed for those facilities using cation resins of a variant base like as lithium or barium.
The key point of this elaborate is the realization which a calculated, or theoretical, relationship does exist, and measurements which vary appreciably from the theoretical values should be investigated and corrective action taken.
Excessively high conductivity stages are an indication of the presence of undesired ions. That condition warrants further investigation to locate the source of the impurity since, further to other chemistry problems; it contributes to common corrosion through increasing the reaction rates of the electrochemical cells. The purity of the makeup water, and a few pH control agents added, should be verified to determine a cause. The pH should also be checked since of the relationship of these parameters. Other chemistry parameters should also be checked, like as Cl- and F-. After the cause of high conductivity has been determined, suitable steps should be taken to return conductivity to its general value. One method that is frequent used is a feed and bleeds process whereby water is added to and drained from the facility at the similar time. Verification of makeup water purity must be ensured to avoid compounding the problem if this method is used.
Low conductivity is also an indicator of a potential problem since, in high purity primary systems, the only probable cause of low conductivity is a low pH. For instance, with a system using high pH ammonium hydroxide control, the preface of air into the facility could result within the formation of nitric acid (HNO3) along with a reduction in pH through the following reaction.
2 N2 + 5 O2 + 2 H2O ↔ 4 HNO3 (3-16)
Conductivity decreases even more than would be expected since of the configuration of NH4NO3. NO3- is not as conductive as OH-, so the NH4NO3 results in a lower conductivity than NH4OH. This neutralization of NH4OH is shown through the following reaction.
NH4OH + HNO3 → NH4NO3 + H2O (3-23)
The water formed is just slightly ionized, and then the solution conductivity is lowered even additional.
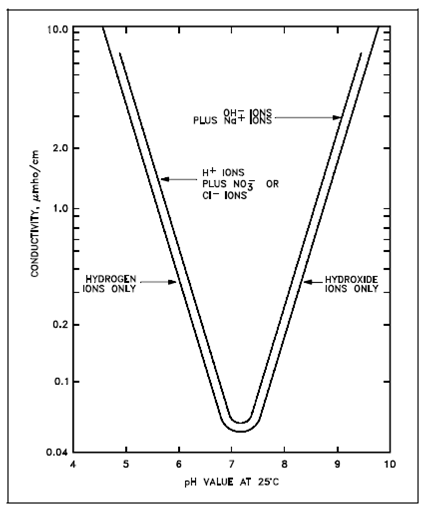
Figure: Theoretical Conductivity as a Function of pH