Molarities of the acid:
Figure gives the log- log plots of [Alamine - 336] vs distribution ratio of cadmium from different molarity of hydrochloric acid. The slopes of the straight lines correspond to 1.82, 1.90 and 1.98 at 0.25, 2.0 and 6.0 M HCl, respectively. This suggests that at all the three different molarities of the acid, the extracting species is CdCl 2-4 .
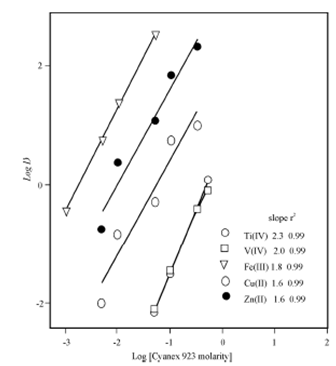
Figure: Effect of Cyanex 923 concentration on the extraction of Ti (IV), V(IV) Fe(III), Cu(II), and Zn (II). Conditions: [Metal ion] = 1x 10-3 (M); [HCl] = 5M
Figure shows the variation in the partitioning of Ti (IV), V (IV), Fe (III), Cu (II) and Zn (II) at 5 M HCl using Cyanex 923 solution of varying (0.001 - 0.5 M) concentration. The extraction increases with the increasing extractant concentration and the log - log plots give a slope of around 2 for all the metal ions. This means that two extractant molecules are involved in the formation of the extracting species.
Sometimes, the results of this slope analysis are not very straight forward and conclusive. These slopes may sometimes suggest the extraction of more than one species.